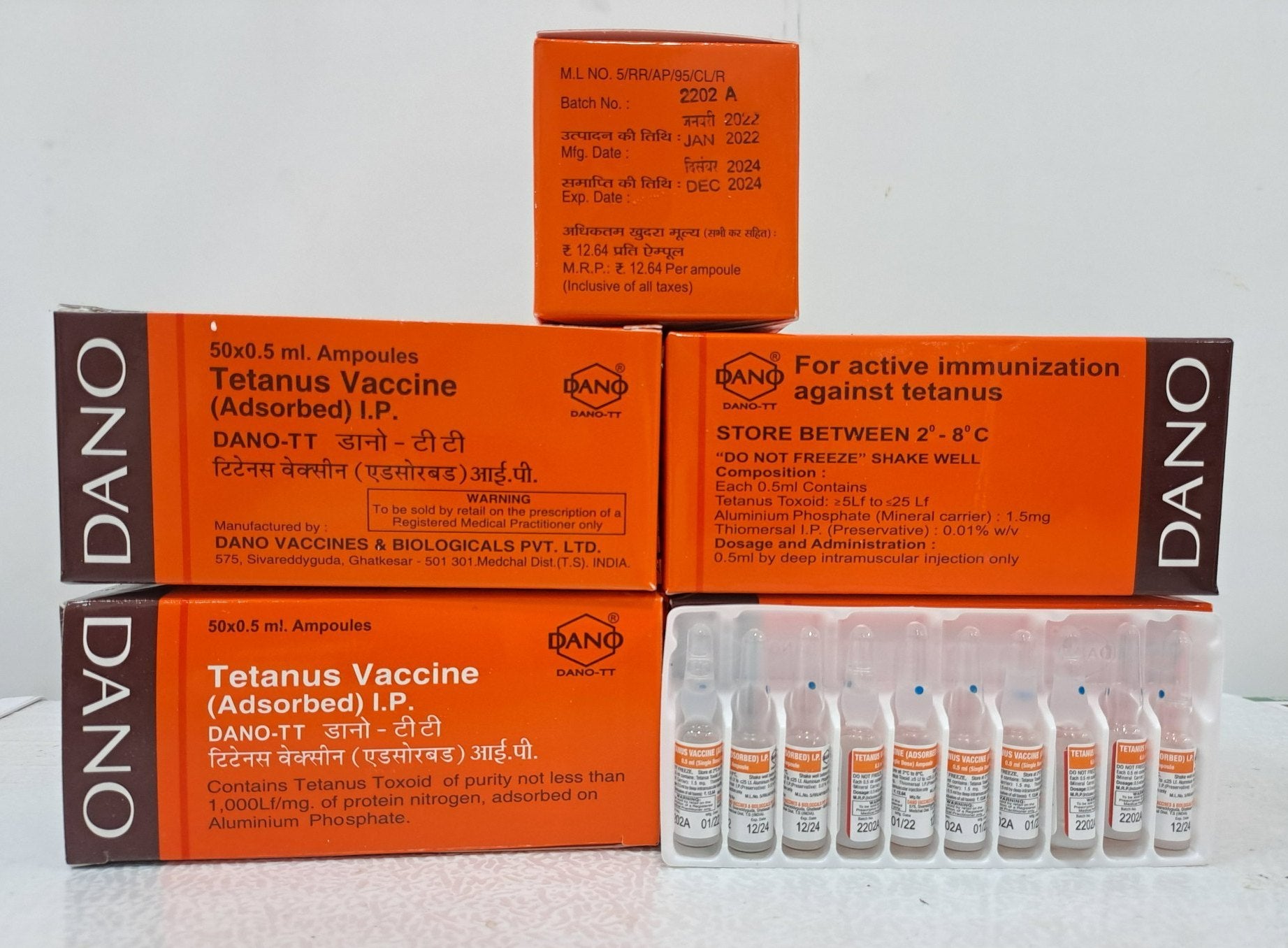
Dano TT – Tetanus Toxoid Adsorbed Vaccine (I.P.)
For Active Immunization Against Tetanus | WHO-Recommended | Pregnancy-Safe
Dano TT is a purified Tetanus Toxoid Adsorbed Vaccine, used for active immunization against tetanus in both children and adults. Manufactured by Dano Vaccines & Biologicals Pvt. Ltd., this vaccine is essential in preventing tetanus and neonatal tetanus, and is widely used in national immunization programs.
🩺 Indications
Prevents tetanus infection (lockjaw) in unvaccinated or inadequately immunized individuals
Essential for maternal immunization to protect both mother and newborn
Used for wound management in individuals at risk
Part of routine immunization schedules across all age groups
💉 Product Details
Name: Dano TT (Tetanus Toxoid Adsorbed)
Formulation: Suspension for Injection (0.5 mL single dose or 5 mL multi-dose vial)
Appearance: Whitish turbid liquid – Shake well before use
Type: Adsorbed Tetanus Toxoid (with Aluminum Phosphate as adjuvant)
Preservative: Thiomersal 0.01% w/v
Storage: 2°C to 8°C (Refrigerated). Do not freeze
Expiry: 2027
Packaging: Available in 0.5 mL ampoules and 5 mL multi-dose vials
📋 Immunization Schedule
| Group | Recommended Doses |
|---|---|
| Children & Adolescents | 2 doses (≥4 weeks apart), 3rd dose after 6 months |
| Pregnant Women | 2 doses during pregnancy (2nd dose ≥2 weeks before delivery) |
| Adults (Booster) | 1 booster dose every 10 years |
| Wound Exposure | 1 dose + TIG if not fully immunized |
✅ May be administered during any trimester of pregnancy
🚨 Administration Guidelines
Route: Intramuscular (IM)
Injection Site:
Adults/Older Children: Deltoid muscle
Infants/Young Children: Anterolateral thigh
Note: Avoid gluteal region (risk of nerve injury)
Shake well before use. Discard if vaccine cannot be re-suspended.
Use a new sterile syringe and needle for each injection.
✅ Compatibility
Can be co-administered with:
BCG, Measles, Rubella, Hepatitis B, OPV/IPV, DTaP, Vitamin A
Administered at separate sites with different syringes
⚠️ Side Effects
Mild: Redness, swelling, pain at injection site, low-grade fever
Rare: Headache, fatigue, myalgia, allergic reactions
Severe allergic responses (anaphylaxis) are rare but require immediate medical response
❌ Contraindications
Known allergy to tetanus toxoid or previous dose reactions
Acute illness or high fever
Prior anaphylaxis to any vaccine component
💬 Precautions
Keep epinephrine 1:1000 readily available during administration
May be safely given to HIV-positive individuals following standard schedules
Do not use if vaccine shows signs of particulate matter or discoloration
🚚 Delivery Info
Metro Manila: Same-day delivery (orders before 3:00 PM)
Outside Metro Manila: 1–3 business days (cold-chain courier)
International Bulk Orders: Accepted with verified documentation
💼 Available For
✅ Clinics
✅ LGUs / Health Units
✅ Schools
✅ Maternity & OB-Gyne centers
✅ Pharmacies
✅ NGOs & Public Health Programs